Research led by the Mode of Action Group Offers New Hope Against Deadly Parasitic Disease
Scientists from the WCAIR and De Montfort University uncover how promising compounds work, paving the way for potentially life-saving treatments for Chagas disease.
The protozoan parasite Trypanosoma cruzi causes Chagas disease, an insidious infection that often remains undetected for decades before eliciting severe symptoms and potentially sudden death. As many as 7 million people around the world are living with this “time-bomb” and a further 75 million are at risk of infection. Current treatments are ineffective and can come with severe side effects. New life saving treatments are urgently required.
Medicinal chemists from De Montford University developed a novel series of quinazoline compounds demonstrating promising activity against T. cruzi in vitro and partial efficacy in an acute Chagas murine model. Utilising their drug target deconvolution platform, scientists from the Mode of Action group in the School of Life Sciences determined that the compounds selectively target the ATP-binding pocket of T. cruzi lysyl-tRNA synthetase 1 (KRS1), an enzyme essential for protein synthesis within the parasite. High-resolution, liganded crystal structures of KRS1 indicate key inhibitor binding interactions thus enabling strategies to improve the drug-like properties of the compounds to be devised.
These studies are published in Science Translational Medicine. Susan Wyllie, Professor of Biochemical Pharmacology and Head of the Mode of Action group, said “Understanding precisely how compounds that show potential for treating parasitic infections work is often vital in guiding their development as effective drugs of the future. While we recognise there is still much work to do, we are delighted that our mechanistic studies have identified a novel drug target in T. cruzi that can be exploited for future drug discovery. The information we have provided will help guide the evolution of this chemical series, hopefully bringing us one step closer to a much-needed treatment for this neglected disease. I would particularly like to highlight the work of Dr Lindsay Tulloch in my lab who did a fantastic job in driving and coordinating this complex research project”.
This research was supported by funding from the Wellcome Trust. The paper can be found here.
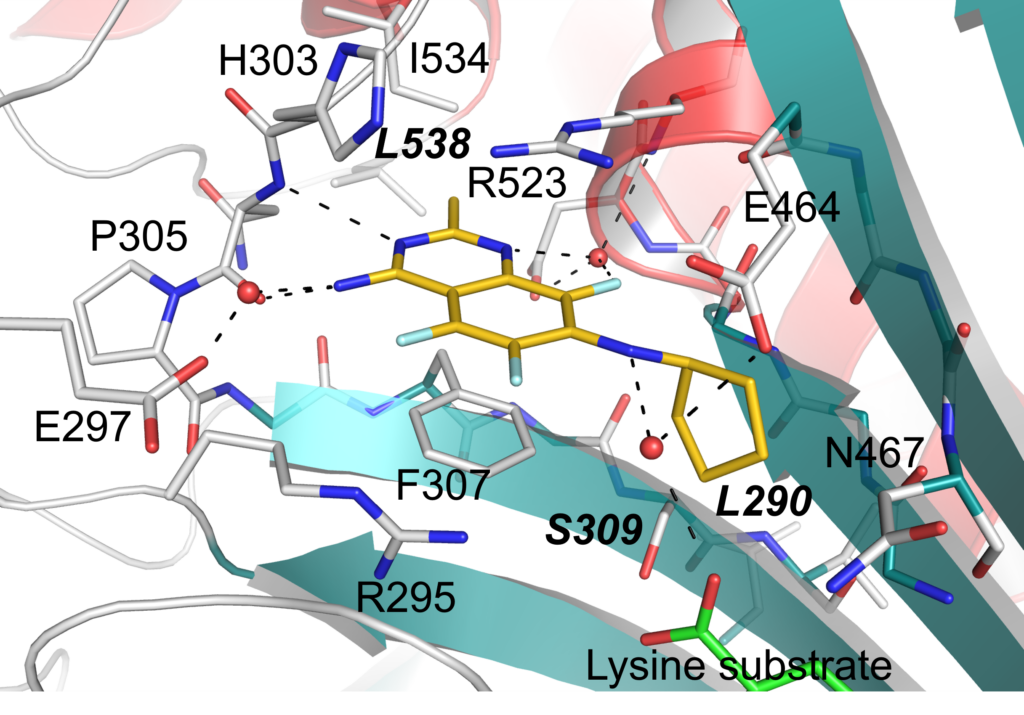
Shown is the crystal structure of TcCpKRS1 with DMU371 (yellow) bound to the active site in the presence of lysine substrate (green). Water molecules are indicated in red, and hydrogen bonding interactions formed with water molecules are represented as black dashed lines. Key residues are labelled using CpKRS1 numbering.